ADVERTISEMENT
Assessing The Potential Of Insoles And Sensory Substitution For Patients With Diabetes
Due to its effects on balance and gait, diabetic peripheral neuropathy can lead to hospitalization and complications for patients. These authors discuss changing perspectives on neuroplasticity and the evolution of sensory substitution, and how these trends have led to wearable technology with potential benefits in this high-risk population.
An estimated 29 million Americans, approximately 10 percent of the adult population, have diabetes and 1.4 million new cases are diagnosed each year.1 Diabetes is the seventh most common cause of death in the United States. Each year, this disease carries a financial burden of $245 billion in medical costs and lost productivity.1 Individuals with diabetes are at an increased risk of myriad complications, including hypertension, cardiovascular disease, vision problems, kidney disease and peripheral neuropathy. The growing impact of diabetes on society highlights the need to find ways to improve treatment, enhance prevention or manage symptoms.
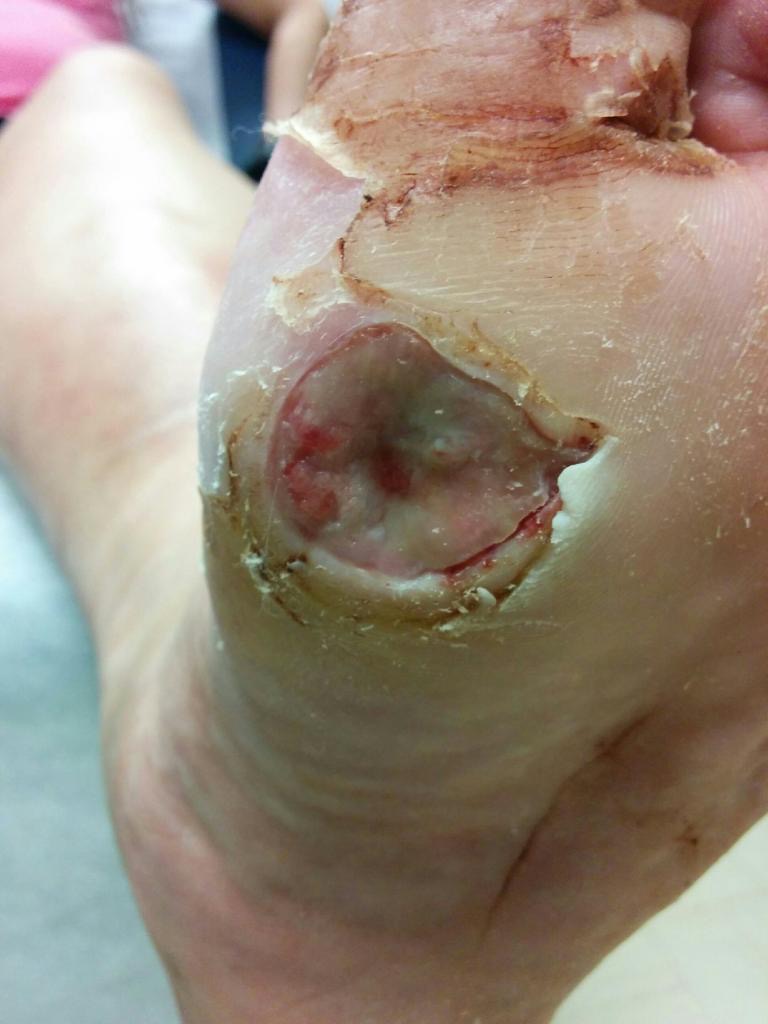 Of all complications of diabetes, it is those of the lower extremity that pose the greatest risk for hospitalization. Central to the causes of lower extremity pathology is diabetic peripheral neuropathy.2 This damage results in impaired functioning of the nerves that provide the commands or impulses to control muscles (i.e., motor nerves) or provide information about pain, touch or position sense (i.e., sensory nerves). The sensory neuropathy component of this disease results in reduced afferent sensory perception in a distal to proximal fashion.
Of all complications of diabetes, it is those of the lower extremity that pose the greatest risk for hospitalization. Central to the causes of lower extremity pathology is diabetic peripheral neuropathy.2 This damage results in impaired functioning of the nerves that provide the commands or impulses to control muscles (i.e., motor nerves) or provide information about pain, touch or position sense (i.e., sensory nerves). The sensory neuropathy component of this disease results in reduced afferent sensory perception in a distal to proximal fashion.
This compromised sensory perception could lead to two troublesome symptoms. First, a lack of sensory afferents could prevent a person from receiving information about plantar pressure sensation (i.e., sensory information from the bottom of the feet). In the event that patients with diabetes lose plantar sensory information, they may keep pressure on a localized area of the foot, leading to tissue ischemia and ulceration.3
Second, aside from poor pain perception and wound predisposition, peripheral neuropathy puts patients at higher risk for impaired balance and gait.4 Typically, balance and gait require the integration of visual, somatosensory and vestibular afferent information.5 Studies have linked the degree of sensory loss with impairment in the stability of balance and gait.4,6,7 Accordingly, the loss of somatosensory afferents from peripheral neuropathy results in an increased risk of ulceration and deteriorating balance and gait control.
Assessing Current Interventions To Address Gait And Balance Issues For Patients With Diabetic Peripheral Neuropathy
If people with diabetes develop gait and balance control issues as a result of peripheral neuropathy, they may be at an increased risk of developing other sequelae, including an increased risk of falling.7-9 After experiencing a fall or being subject to balance control issues, people with diabetes may not feel safe while ambulating, resulting in reduced activity levels and an increased reliance on walking aids.
Therefore, it is critical that we find new ways to dose activity and improve balance and gait control in these individuals. Researchers have attempted to improve gait and balance control in these individuals using interventions such as physiotherapy, strength training, postural control training or yoga.
For instance, investigators have had people with diabetes perform lengthy intervention programs involving physiotherapy-guided training.10 This type of intervention resulted in moderate improvements in gait and balance control with mixed effects. Other investigators have specifically used a postural control training intervention to improve balance control in patients with diabetes, and have demonstrated some degree of success with these measures.11 Based on the fact that reduced muscle strength may lead to an increased risk of falling in patients with diabetes, researchers have had individuals perform strength training exercises to improve gait and balance with modest results.12,13
Custom orthotics are another avenue for ambulation symptom management in patients with peripheral neuropathy and researchers have demonstrated that custom orthotics can enhance balance control in individuals with neuropathy.14
Although these aforementioned interventions may show moderate improvements, there are barriers to these interventions such as expertise requirement, transportation difficulties, scheduling issues, high time commitments and lack of accessibility. Due to these complications, it may be difficult for these individuals to keep up the training intervention, causing them to return to their baseline activity levels, ultimately not changing their risk of falls and continued disease progression. The evidence suggests that the current methods to improve balance and gait control in individuals with diabetes are not very fruitful and further methods need to emerge to help these individuals.
Emerging Research And Changing Views On Neuroplasticity
One emerging method for improving rehabilitation for those with certain diseases or disorders is leveraging neuroplasticity. In general, neuroplasticity is the ability of the central nervous system to change or “re-wire.” These changes can take the form of altering connections between individual neurons (synaptic) or within a neuron itself (non-synaptic).15 After someone has experienced an injury or there is a gradual deterioration of the nervous system, neuroplasticity allows for the reforming of this system to help compensate for these changes.
 Physicians originally thought the central nervous system, once developed, was static or remained unchanged. One pivotal study using an animal model challenged this belief and revealed that the adult central nervous system is not as static as once thought.16 This landmark study revealed that the representation of the digits within the somatosensory areas of the brain can change drastically depending on whether the digits were constrained to act together as one or function independently, elucidating the adaptability of the adult central nervous system.
Physicians originally thought the central nervous system, once developed, was static or remained unchanged. One pivotal study using an animal model challenged this belief and revealed that the adult central nervous system is not as static as once thought.16 This landmark study revealed that the representation of the digits within the somatosensory areas of the brain can change drastically depending on whether the digits were constrained to act together as one or function independently, elucidating the adaptability of the adult central nervous system.
Since this pivotal research study, a plethora of work has been dedicated to understanding the process of neuroplasticity following impairment of central nervous system function. Emerging research has focused on how the brain changes following a central nervous system injury and this scope of research seems promising for implementing this knowledge for creating neural repair interventions.17 Further research, however, is necessary to translate the basic science to clinical practice and improve the effectiveness of treatment programs for rehabilitation.
Leveraging Neuroplasticity With Sensory Substitution
A number of different treatment methods to improve motor function have emerged based on the knowledge of neuroplasticity. These treatment approaches include constraint-induced movement therapy, repetitive brain stimulation or pharmacology interventions paired with behavioral tasks. These methods focus primarily on the motor side of rehabilitation as our ability to perform very precise movements and ambulate safely in our everyday lives depend on a properly functioning motor system. What we often forget is the importance of the sensory system in controlling these movements. Clinical observations and motor control theories all point to the fact that when the sensory system fails, the motor system becomes impaired and ultimately affects our quality of life.18
If a person has sensory integration problems, it is apparent that there is a need to find a health solution to improve sensory processing as a means to improve motor control in these individuals. Neural repair of sensory afferents, such as regenerating nerves, may seem like a promising option but we are still decades away from implementing this method as a health solution. A much more promising solution is to substitute a lost sense with another form of sensory input, also known as sensory substitution. Sensory substitution builds on the science of neuroplasticity. Due to neuroplasticity, the central nervous system is able to use a new type of sensory input (e.g., artificial vibrotactile input) and associate this input with the features of the previous lost sense.
In recent years, there have been numerous attempts to restore sensory function by a sensory substitution system. These sensory substitution systems have helped replace audition, vision and vestibular senses.19-21 While most sensory substitution systems to date involve visual substitution, there has recently been successful implementation of a vibrotactile sensory substitution system. These vibrotactile sensory substitution systems can improve balance control in people with vestibular dysfunction, provide a person’s orientation via inputs from a compass or reroute sensory information from fingertips with sensory loss to neighboring locations to help guide/control hand movements.19,22,23
It seems apparent that somatosensory inputs are quite effective for a sensory substitution system to improve motor function. In the example of individuals with diabetes who have peripheral neuropathy, there is impaired somatosensory function that progresses in a distal to proximal manner. Since the sensation from the feet is one of the first areas to degrade in peripheral neuropathy, it would influence the perception of plantar pressure and impact the ability of these patients to walk safely.
Therefore, there is a need to improve, restore or replace inputs regarding plantar pressure proprioception to improve the motor control of gait and balance in these individuals. The increasing presence of wearable technologies in our day-to-day lives will extend from its present, consumer-focused applications to more medically-oriented products that have the ability to parlay sensory information in new ways that could improve outcomes in many disease states, especially diabetes.
 Could New Wearable Technologies Help Replace Lost Or Impaired Plantar Pressure Proprioception?
Could New Wearable Technologies Help Replace Lost Or Impaired Plantar Pressure Proprioception?
The SurroGait Rx™ Sensory Substitution System (Orpyx Medical Technologies) may improve gait and balance in individuals with reduced or absent plantar pressure sensation. With lost sensation in the feet, it becomes difficult to detect changes in posture. This ultimately impacts a person’s ability to stand or walk. SurroGait Rx technology effectively replaces the lost or impaired plantar pressure proprioception with a new type of sensory input.
The SurroGait Rx was designed to help people with impaired foot sensation to be aware of their base of support limits. The SurroGait Rx functions by placing pressure sensing insoles in the shoes of patients to detect when they are approaching the limits of their base of support. Similar to how the “rumble strips” inform people about where they are in their own lane while driving, the SurroGait Rx uses information from the pressure sensing insoles to tell people about their base of support via vibrations from a vest worn on the back.
Research has shown that the SurroGait Rx has beneficial effects on gait and posture control in individuals with peripheral neuropathy. In a small scale clinical trial, researchers sought to determine if there were changes in measures of gait and balance control after patients used the SurroGait Rx device for an extended period of time.24 Specifically, researchers obtained baseline gait and balance control measures from 10 patients with peripheral neuropathy before wearing the SurroGait Rx device for four weeks. These baseline measures included 1) center of pressure path length to assess balance, 2) spatial/temporal stride and step characteristics to determine gait kinematic differences and 3) kinetic variable of gait.
In terms of balance control, patients with peripheral neuropathy showed less sway when balancing with their eyes closed, suggesting improved balance control.24 These changes occurred because of the demand to incorporate somatosensory input to guide balance control in the absence of vision. Given that patients with peripheral neuropathy already have compromised plantar pressure proprioception, they likely relied on the vibrotactile inputs from the SurroGait Rx device to improve their perception of their swaying body and accordingly improved their balance.
In terms of gait measures, the SurroGait Rx device also shows beneficial effects.24 When wearing the device, patients with peripheral neuropathy had increased stride frequency (i.e., more steps taken over a fixed distance) and reduced variability of stride frequency (i.e., keeping the number of steps taken over a fixed distance consistent). Overall, the evidence suggests there is a positive benefit of the SurroGait Rx for patients with peripheral neuropathy in the form of immediate improvements in gait and balance.
Outside of direct sensory substitution-based feedback, there is also a role to play for cue-based feedback to patients with diabetic peripheral neuropathy. Another technology, the SurroSense Rx™ smart insole system (Orpyx Medical Technologies) does this by providing offloading and activity guidance based on data collected in a pressure sensitive insole. While the product’s impact with respect to gait and balance is less pronounced, the simplicity of the system — with its inconspicuousness and potential ease of deployment — provides a high degree of promise in its own right.
In Conclusion
With the increasing acceptance of wearable technologies, we are encouraged that we will continue to see the translation of consumer-based technology to medically-oriented technology that carries the promise of improving gait and balance while saving both limbs and lives.
Dr. Asmussen is a Postdoctoral Fellow with the Faculty of Kinesiology at the University of Calgary in Calgary, Alberta.
Dr. Everett is a resident in the Department of Surgery, Section of Plastic Surgery at the University of Calgary in Calgary, Alberta. She is the CEO and Co-Founder of Orpyx Medical Technologies in Calgary.
Dr. Armstrong is the Director of the Southern Arizona Limb Salvage Alliance and a Professor of Surgery at the University of Arizona Medical Center in Tucson, Ariz.
References
- American Diabetes Association. Statistics about Diabetes. Available at https://www.diabetes.org/diabetes-basics/statistics/ . Published June 10, 2014.
- Cakici N, Fakkel TM, van Neck JW, Verhagen AP, Coert JH. Systematic review of treatment for diabetic peripheral neuropathy. Diabet Med. 2016; epub Jan. 29.
- Cavanagh PR, Simoneau GG, Ulbrecht JS. Ulceration, unsteadiness, and uncertainty: the biomechanical consequences of diabetes mellitus. J Biomech. 1993; 26(Suppl1):23–40.
- Ducic I, Kelly S, Lee DA. Relationship between loss of pedal sensibility, balance, and falls in patients with peripheral neuropathy. Ann Plast Surg. 2004; 52(6):535-40.
- Brandt T. Sensory function and posture. In Ambrald B, Berthoz A, Clarac F (eds.) Posture and Gait: Development, Adaptation, and Modulation. Experta Medica, New York, 1998, pp. 137-55.
- Courtemanche R, Teasdale N, Boucher P, et al. Gait problems in diabetic neuropathic patients. Arch Phys Med Rehab. 1996; 77(9):849-855.
- Wallace C, Reiber GE, LeMaster J, Smith DG, Sullivan K, Hayes S, Vath C. Incidence of falls, risk factors for falls, and fall-related fractures in individuals with diabetes and a prior foot ulcer. Diabetes Care. 2002; 25(11):1983-6.
- Schwartz AV, Hillier TA, Sellmeyer DE, Resnick HE, Gregg E, Ensrud KE. Older women with diabetes have a higher risk of falls. Diabetes Care. 2002; 25(10):1749-54.
- Tiling LM, Darawil K, Britton M. Falls as a complication of diabetes mellitus in older people. J Diabetes Complications. 2006; 20(3):158-162.
- Allet L, Armand S, de Bie RA, Golay A, Monnin D, Aminian K. The gait and balance of patients with diabetes can be improved: a randomised controlled trial. Diabetologia. 2010; 53(3):458-466.
- Morrison S, Colberg SR, Parson HK, Vinik AI. Relation between risk of falling and postural sway complexity in diabetes. Gait Posture. 2012; 35(4):662-668.
- MacGilchrist C, Paul L, Ellis BM, Howe TE, Kennon B, Godwin J. Lower-limb risk factors for falls in people with diabetes mellitus. Diabet Med. 2010; 27(2):162-168.
- Richardson J, Thies SB, DeMott TK, Ashton-Miller JA. A comparison of gait characteristics between older women with and without peripheral neuropathy in standard and challenging environments. J Am Geriatr Soc. 2004; 52(9):1532-37.
- Croarkin E, Eisenfeld R, Zampieri C, Rekant J. Custom orthotics to mitigate effects of chemotherapy-induced peripheral neuropathy. Rehabil Oncol. 2015; 33(1):43-50.
- Jessel T, Kandel E, Siegelbaum S, Schwartz J, Hudspeth AJ. Principles of Neural Science. McGraw-Hill, New York, 2012, pp. 1165-1328.
- Wang X, Merzenich MM, Sameshima K, Jenkins WM. Remodelling of hand representation in adult cortex determined by timing of tactile stimulation. Nature. 1995; 378(1):71-75.
- Nudo R. Recovery after brain injury: mechanism and principles. Front Hum Neurosci. 2013; 7:887.
- Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nature Neurosci. 2002; 5(11):1226-1235.
- Horak FB, Dozza M, Peterka R, Chiari L, Wall C. Vibrotactile biofeedback improves tandem gait in patients with unilateral vestibular loss. Ann NY Acad Sci. 2009; 1164:279-81.
- Lee VK, Nau AC, Laymon C, Chan KC, Rosario BL, Fisher C. Successful tactile based visual sensory substitution use functions independently of visual pathway integrity. Front Hum Neurosci. 2014; 8:291.
- Lobo L, Travieso D, Barrientos A, Jacobs DM. Stepping on obstacles with a sensory substitution device on the lower leg: practice without vision is more beneficial than practice with vision. PLoS One. 2014; 9(6):e98801.
- Nagel SK, Carl C, Kringe T, Martin R, Konig P. Beyond sensory-substitution –learning the sixth sense. J Neural Eng. 2005; 2(4):R13-R26.
- Cipriani C, D’Alonzo M, Carrozza MC. A miniature vibrotactile sensory substitution device for multifingered hand prosthetics. IEEE Trans Biomed Engineer. 2011; 59(2):400-408.
- Bauman J, LeVangie M, Nigg SR, Everett B, Nigg BM. Improving neuropathic gait and balance via sensory substitution. Presented at the 39th Annual Meeting of the American Society of Biomechanics, August 5-8, 2015, Columbus, OH. Available at https://www.asbweb.org/conferences/2015/abstracts/PD6E_3--Improving%20Neuropathic%20Gait%20And%20Balance%20Via%20Sensory%20Substitution--%28Bauman%29.pdf .











