ADVERTISEMENT
A Closer Look At Drilling And Cartilage Replacement For Talar Dome Lesions
 While there are indications for a variety of reparative or restorative techniques for talar dome lesions, these authors review the literature and advantages of retrograde drilling and juvenile particulated cartilage for these complex lesions, and provide two illustrative case studies.
While there are indications for a variety of reparative or restorative techniques for talar dome lesions, these authors review the literature and advantages of retrograde drilling and juvenile particulated cartilage for these complex lesions, and provide two illustrative case studies.
Osteochondral lesions can be a severely debilitating condition that physicians frequently miss due to subtle initial radiographic findings. Symptoms may include pain, swelling, locking, popping and catching within the ankle following a single or multiple traumatic incidents. Transchondral lesions involve only the articular cartilage of the talar dome while osteochondral lesions involve the cartilage and the subchondral bone of the talar dome.1 Historically, commonly used terminology to denote these lesions includes flake fracture, intra-articular fracture of the talus and osteochondritis dissecans.2
We can categorize surgical treatment options for osteochondral lesions of the talus into reparative techniques and restorative techniques. Reparative techniques generally involve debridement and bone marrow stimulation with production of fibrocartilage to replace native hyaline cartilage. Restorative techniques attempt to replace the damaged hyaline cartilage with hyaline cartilage of various forms.
While there are certainly indications for nearly all the aforementioned techniques, this article attempts to highlight advantages of retrograde drilling (that are distinct from prograde drilling) as well as the use of juvenile particulated cartilage in the treatment of these complex lesions.
What The Literature Reveals About Retrograde Drilling
The technique of bone marrow stimulation, whether prograde or retrograde, is essential to successful management of these lesions. Conti, Taranow and their respective coauthors first described retrograde drilling or transmalleolar drilling of talar dome lesions in the 1990s.3,4 With an overall patient satisfaction of 81 percent, Taranow and colleagues did not find any statistically significant difference in results with retrograde drilling among stage II, III and IV lesions. Stage I includes subchondral bone compression or bone bruise, Stage II involves subchondral cysts that are not acute, Stage III includes partially separated or detached fragments, and stage IV includes displaced fragments.3,5 With radiographic healing noted in 88 percent, Taranow and colleagues recommended that one could successfully treat Stage I and Stage II lesions with retrograde drilling.3 Rosenberger, Kono and their respective coworkers similarly reported a success rate of 88 percent for retrograde drilling.6,7
Zengerink and coworkers completed a meta-analysis of surgical treatment options for talar dome lesions in 2010.8 Regarding retrograde drilling, the authors did not make a reliable conclusion due to the limited number of higher-level studies concerning retrograde drilling. However, they recommended using retrograde drilling for Berndt and Hardy Type I and Type II lesions with intact articular cartilage.
There is a consensus in the foot and ankle surgical community that lesions < 1.5 cm2 may respond well to bone marrow stimulation. Chuckpaiwong, Choi and their respective colleagues wrote highly referenced articles to substantiate this fact.9,10 However, these studies evaluate prograde drilling of the talus or both microfracture and abrasion arthroplasties. To this date, there have not been any studies that have measured and defined a size limit that correlated to poor outcomes for retrograde drilling. The majority of authors agree that one can use retrograde drilling for defects in subchondral bone with intact and viable articular cartilage with or without a large cyst, in which case a cancellous bone graft might be required in addition to drilling.
Comparing Retrograde With Antegrade Drilling
Kono and coworkers sought to determine whether antegrade drilling or retrograde drilling had more effective outcomes as determined by American Orthopaedic Foot and Ankle Society (AOFAS) scores and arthroscopic follow-up evaluation.7 At one year, all 19 patients who received antegrade drilling had deteriorated tibial cartilage by one grade (Pritsch classification) while the use of retrograde drilling on 11 lesions resulted in no changes.7,11 In arthroscopic evaluation of the lesions, surgeons only saw improvement in those who received retrograde drilling (27.2 percent) and 72 percent of lesions were unchanged within this group. Within the group who received antegrade drilling, 42.1 percent had evidence of cartilage deterioration. While AOFAS scores were not significantly different between the two groups, the authors “strongly recommend avoiding articular cartilage to prevent the possibility of subsequent degeneration.”
 Takao and colleagues similarly found deterioration of articular cartilage one year after initial surgery involving antegrade drilling in all patients with previously intact cartilage.12 These authors assessed efficacy as measured by magnetic resonance imaging (MRI), arthroscopy and AOFAS scores at a mean follow-up of 39 months. The study focused on drilling in three situations: chondral, subchondral or a combined injury. Most importantly, upon arthroscopic visualization in the subchondral group, all 10 cases demonstrated deterioration with eight deteriorating by one grade and two deteriorating by two grades. These authors recognize the potential undesired consequences of articular cartilage damage with antegrade drilling and suggest retrograde drilling as an alternative.
Takao and colleagues similarly found deterioration of articular cartilage one year after initial surgery involving antegrade drilling in all patients with previously intact cartilage.12 These authors assessed efficacy as measured by magnetic resonance imaging (MRI), arthroscopy and AOFAS scores at a mean follow-up of 39 months. The study focused on drilling in three situations: chondral, subchondral or a combined injury. Most importantly, upon arthroscopic visualization in the subchondral group, all 10 cases demonstrated deterioration with eight deteriorating by one grade and two deteriorating by two grades. These authors recognize the potential undesired consequences of articular cartilage damage with antegrade drilling and suggest retrograde drilling as an alternative.
Why You Should Think Twice About Osteochondral Allograft Transplantation
Restorative techniques encompass some form of grafting. These include osteochondral autologous and allograft transplantation, chondrocyte implantation and juvenile articular cartilage implantation. Traditionally, one considers restorative techniques only after failed bone marrow stimulation techniques and with lesions over 1.5 cm2, a depth of 5 mm and with underlying cysts.13,14
Osteochondral autologous transplantation surgery (OATS) may be the least favorable of the restorative techniques. Outerbridge and colleagues first described it in the knee in 1995, defining OATS as transferring an osteochondral graft from a non-weightbearing region to a chondral defect.15 It is an open technique requiring a malleolar osteotomy and harvesting cartilage at the ipsilateral knee.13 While addressing both bone and cartilage pathology, there is no risk of disease transmission or transplant rejection, and the longevity of cartilaginous cells is higher.13
However, donor site morbidity, pain and size limitations are disadvantages.13,14 The topography and morphology of the cartilage at the knee and talus are significantly different.16 In addition to opposite topography (concave versus convex), the average thickness of the talar articular cartilage is approximately 0.89 mm versus 6 mm at the knee.16
Most recently, Yoon and colleagues compared outcomes of osteochondral autologous transplantation and repeat arthroscopy in a level 3 study.17 At a mean follow-up of 50 months, 81.8 percent of patients receiving OATS achieved AOFAS scores greater than 80 versus 31.8 percent of the patients receiving arthroscopic revision.
While potential benefits of talar allograft transplantation include the ability to reconstruct lesions of any size and circumventing donor site morbidity, the evidence is poor to advocate for osteochondral allograft transplantation.18 Cell viability reportedly decreases by 30 percent after 28 days and as most tissue banks require one month of screening for disease, the attraction of this procedure diminishes.16 In a 4.1-year follow-up of 17 ankles (16 of which had failed previous surgeries), Haene and coworkers reported five failures and two requiring reoperation with only four ultimately being symptom-free.19 They advised that this procedure might improve functional outcomes in a carefully evaluated patient. Equally important are cost, risk of disease transmission and contour variance as these factors further tarnish allograft transplantation as a surgical option.13
Current Insights On The Advantages Of Cultured Grafts
Cultured grafts, including both chondrocyte implantation and particulated juvenile cartilage, are newer and superior techniques. Brittberg and colleagues originally described autologous chondrocyte implantation in the knee in 1994.20 Giannini and colleagues then successfully used this method to treat osteochondral lesions of the talus in 2001.21
 There are several generations of chondrocyte implantation including autologous chondrocyte transplantation, matrix-induced autologous implantation and matrix-induced autologous chondrocyte transplantation, whereby the cultured cells are embedded into a three-dimensional collagen matrix.14 These are two-stage procedures that involve culturing cartilage, most commonly from the knee. A meta-analysis of autologous chondrocyte implantation in the ankle found there was a lack of level I studies to conclude that autologous chondrocyte implantation was superior or inferior to comparable techniques despite initial success rates close to 90 percent.22 Major drawbacks of this procedure include the fact that it is a two-stage procedure as well as its cost.
There are several generations of chondrocyte implantation including autologous chondrocyte transplantation, matrix-induced autologous implantation and matrix-induced autologous chondrocyte transplantation, whereby the cultured cells are embedded into a three-dimensional collagen matrix.14 These are two-stage procedures that involve culturing cartilage, most commonly from the knee. A meta-analysis of autologous chondrocyte implantation in the ankle found there was a lack of level I studies to conclude that autologous chondrocyte implantation was superior or inferior to comparable techniques despite initial success rates close to 90 percent.22 Major drawbacks of this procedure include the fact that it is a two-stage procedure as well as its cost.
With healthcare expenditure a topic of concern in this present day and at a cost of one-fifth that of autologous chondrocyte implantation, particulated juvenile cartilage is an attractive treatment option gaining popularity. Commercially available since 2007, the DeNovo NT Natural Tissue Graft (Zimmer) consists of particulated juvenile cartilage. The fragments are from donors younger than 13 years of age with over 50 percent of donors being less than three years of age.23 Of much higher importance than cost alone, the younger cells confer a greater advantage in comparison to aged adult cells by having a higher potential for cell division and matrix production expressing greater levels of mRNA for aggrecan and collagens type II and IX. Viable cells in juvenile cartilage reach four times that of adult cartilage. The cells are also immune privileged and therefore do not mount an immunogenic reaction.23
Given the relatively recent introduction of DeNovo NT to the market, few long-term clinical trials have occurred and even fewer reports on the use of the modality in the ankle. Coetzee and colleagues studied the outcomes of pain and function using particulated juvenile cartilage on 24 ankles, 14 of which had failed previous bone marrow stimulation.24 In the study, the lesion size was greater than 10 mm and lesions were separated into moderate and larger size lesions with 15 mm being the cutoff between the two. At an average follow-up of 16 months, those achieving an AOFAS score of > 80 included 92 percent of those with moderate-sized lesions (12/13). The value of the bone grafting in addition to cartilage implantation is unknown. In this series, 67 percent of ankles with deep lesions > 6 mm that did not have concomitant grafting at the time of surgery demonstrated poor or fair AOFAS scores (<80).
Kruse and coworkers reported on one arthroscopically treated lesion measuring 7 mm x 5 mm in a 30-year-old woman.25 At the two-year follow-up, the patient was pain-free with a return to desired activity.
Juvenile cartilage graft is especially advantageous for chondral injuries. Choi and coworkers found a significant difference in the peak age distribution between chondral and osteochondral type injuries to the talus (50s versus the 20s respectively).9 Skeletal maturity causes failure to occur at the junction of the well calcified and non-calcified layer. In addition, chondral lesions in these older individuals demonstrated a significantly greater duration of symptoms and a higher presence of subchondral cysts.
Several authors have reported less favorable outcomes of marrow stimulation procedures for the treatment of osteochondral defects in older patients.10,21 In addition, there is an age-related decline in the number of mesenchymal stem cells in the talar bone marrow.26 As such, the use of juvenile allograft is more appropriate.
Case Study One: Retrograde Drilling For Severe Ankle Pain
A 50-year-old male presented to the clinic with a referral for surgical management for a painful right ankle that was unresponsive to conservative care, including bracing and casting, for several years. He had sustained multiple sprains to this ankle and also had a history of an open fracture complicated with osteomyelitis. At the time of the visit, the patient had treatment from a pain management specialist for his pain, which he described as level 10.
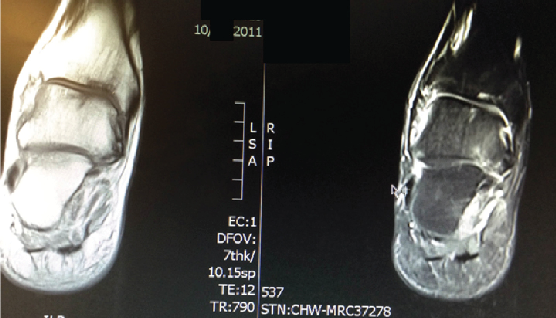
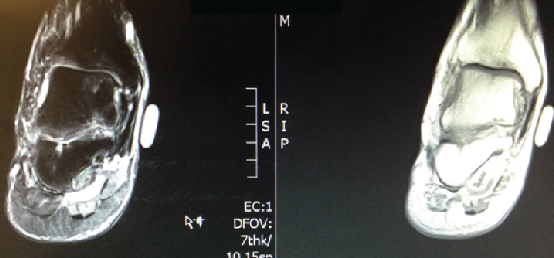 The patient’s physical exam was significant for tenderness to palpation across the ankle joint with pain on active and passive range of motion. He was neurovascularly intact. The patient had clinical and radiographic evidence of a 1.4 cm osteochondritis dissecans as shown in an MRI from March 2011.
The patient’s physical exam was significant for tenderness to palpation across the ankle joint with pain on active and passive range of motion. He was neurovascularly intact. The patient had clinical and radiographic evidence of a 1.4 cm osteochondritis dissecans as shown in an MRI from March 2011.
After injections only alleviated his pain for one day, we decided to perform arthroscopically assisted retrograde drilling in July 2011. The patient wore a weightbearing controlled ankle motion (CAM) walker with limited time in dependency for four weeks followed by four weeks of physical therapy. He subsequently described his pain level as level 2 at the end of September. By October, the patient was functioning normally as he was walking without pain and any bracing.
Case Study Two: Insights On Using Particulated Juvenile Cartilage Allograft
A 69-year-old female got a referral to our office in July 2013 for surgical management of a painful ankle. She had a history of a severe ankle sprain, which had occurred seven years ago, with a resultant osteochondritis dissecans. The patient had been treated with bracing, injections and physical therapy. Although bracing worked at first, it had not worked for a couple of years. The patient’s pain level with an ankle-foot orthotic (AFO) brace was 7.5.
There is a history of osteoarthritis of her wrist as well as low back problems but no other contributory problems. She had an intact neurovascular status. After the consultation, we decided to perform arthroscopic debridement of the osteochondritis dissecans along with using a juvenile cartilage allograft. After performing arthroscopic debridement of the Berndt and Hardy type 4 lesion, we transplanted the particulated juvenile cartilage allograft. This occurs by first form fitting the defect, molding the allograft with fibrin, then using the fibrin and allograft mold to insert this into the defect arthroscopically.
She was non-weightbearing for eight weeks followed by four weeks in a weightbearing CAM walker and subsequent physical therapy. Although her post-op course was uneventful, it wasn’t until October that she finished physical therapy. At the December 2013 visit, she was pain-free, was walking four miles a day, biking in normal weather and working out on an elliptical machine. By December, she was performing her normal activities well beyond what she had done for the past four years and was able to function to the highest level she desired in walking, biking and elliptical training.
This patient did not have any problems or complications a year later when we saw her in December 2014. She is still engaging in activities beyond what she was doing in the five years before her surgery. The patient has continued to increase her overall activity level within the past year.
In Conclusion
In fact, the decision for treating osteochondritis dissecans may still sometimes be resection of the lesion. In those cases, the DeNovo NT graft may be primarily effective. Secondarily, one might consider mosaicplasty or OATS procedures. However, in a non-displaced lesion with intact cartilage, we may consider retrograde drilling primary treatment and reserve resection of the lesion with direct grafting for major defects. Future studies would be helpful to evaluate each of these modalities over a longer period of time.
Dr. Grady is a Fellow of the American Society of Podiatric Surgeons and the American College of Foot and Ankle Orthopedics and Medicine. He is the Director of the Foot and Ankle Institute of Illinois and the Director of the Foot and Ankle Institute for Research (FAIR) in Oak Lawn, Ill. He is also an Attending Surgeon at Advocate Christ Medical Hospital and Medical Center in Oak Lawn, Ill.
Dr. Zager is an Attending Surgeon at Advocate Christ Medical Hospital and Medical Center.
Dr. Trotter is a third-year surgical resident at the Jesse Brown Veterans Affairs Medical Center in Chicago.
Dr. Nagesh is a second-year surgical resident at the Jesse Brown Veterans Affairs Medical Center in Chicago.
References
- Saxena A. Osteochondral lesions of the talus. In: Special Procedures in Foot and Ankle Surgery. Springer, London, 2013.
- Alexander AH, Lichtman DM. surgical treatment of transchondral talar-dome fractures (osteochondritis dessicans). Long-term follow-up. J Bone Joint Surg Am. 1980; 62(4):646-52.
- Taranow WS, Bisignani GA, Towers JD. Retrograde drilling of osteochondral lesions of the medial talar dome. Foot Ankle Int. 1999;20(8):474-80.
- Conti SF, Taranow WF. Transtalar retrograde drilling of medial osteochondral lesions of the talar dome. Operative Techniques in Orthopaedics. 1996; 6:226-230.
- Mcgahan PJ, and Pinney SJ. Current concept review: osteochondral lesions of the talus. Foot Ankle Int. 2010; 31(1):90-101.
- Rosenberger RE, Fink C, Bale RJ, et al. Computer-assisted minimally invasive treatment of osteochondrosis dessicans of the talus. Oper Orthop Traumatol. 2006; 18(4):300–316.
- Kono M, Takao M, Naito K. Retrograde drilling for osteochondral lesions of the talar dome. Am J Sports Med. 2006; 34(9):1450–1456.
- Zengerink M, Szerb I, Hangody L, Dopirak RM, Ferkel RD, van Dijk CN. Current concepts: treatment of osteochondral ankle defects. Foot Ankle Clin. 2006; 11(2):331-59.
- Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: Is there a critical defect size for poor outcome? Am J Sports Med. 2009; 37(10):1974-1980.
- Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy. 2008; 24(1):106-112.
- Pritsch M, Horoshovski H, Farine I. Arthroscopic treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 1986; 68(6):862-865.
- Takao M, Ochi M, Naito K, et al. Arthroscopic drilling for chondral, subchondral, and combined chondral-subchondral lesions of the talar dome. Arthroscopy. 2003; 19(5):5424-530.
- Kadakia AR, Espinosa N. Why allograft reconstruction for osteochondral lesion of the talus? The Osteochondral Autograft Transfer System seemed to work quite well. Foot Ankle Clin N Am. 2013; 18(1):89–112.
- Johnson B, Lever, C, Roberts S, et al. Cell cultured chondrocytes implantation and scaffold techniques for osteochondral talar lesions. Foot Ankle Clin N Am. 2013; 18(1):135–150.
- Outerbridge HK, Outerbridge AR, Outerbridge RE. The use of a lateral patellar autologous graft for the repair of a large osteochondral defect in the knee. J Bone Joint Surg Am. 1995; 77(1):65-72.
- Baums MH, Schultz W, Kostuj T, Klinger HM. Cartilage repair techniques of the talus: An update. World J Orthop. 2014; 5(3):171-179.
- Yoon HS, Park YJ, Lee M, Choi WJ, Lee JW. Osteochondral Autologous Transplantation is superior to repeat arthroscopy for treatment of osteochondral lesions of the talus after failed primary arthroscopic treatment. Am J Sports Med. 2014; 42(8):1896-1903.
- Savage-Elliott I, Ross KA, Smyth NA, Murawski CD, Kennedy JG. Osteochondral lesions of the talus: a current concepts review and evidence based treatment paradigm. Foot Ankle Spec. 2014; 7(5):414-422.
- Haene R, Qamirani E, Story RA, Pinsker E, Daniels TR. Intermediate outcomes of fresh talar osteochondral allografts for treatment of large osteochondral lesions of the talus. J Bone Joint Surg. 2012; 94(12):1105-10.
- Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. New England J Med. 1994; 331(14):889 895.
- Giannini S, Buda R, Grigolo B. Autologous chondrocyte transplantation in osteochondral lesions of the ankle joint. Foot Ankle Int. 2001; 22(6):513-7.
- Niemeyer P, Salzmann G, Schmal H, Mayr H, Sudkamp NP. Autologous chondrocyte implantation for the treatment of chondral and osteochondral defects of the talus: a meta-analysis of available evidence. Knee Surg Sports Traumatol Arthrosc. 2012; 20(9):1696–1703.
- Available at https://www.zimmer.com/medical-professionals/products/biologics-sports-medicine/denovo-nt-natural-tissue.html .
- Coetzee JC, Giza E, Schon LC, et al. Treatment of osteochondral lesions of the talus with particulated juvenile cartilage. Foot Ankle Int. 2013;34(9):1205-1211.
- Kruse DL, Ng A, Paden M, Stone PA. Arthroscopic De Novo NT® juvenile allograft cartilage implantation in the talus: a case presentation. J Foot Ankle Surg. 2012;51(2):218-21.
- Bergman RJ, Gazit D, Kahn AJ, et al. Age-related changes in osteogenic stem cells in mice. J Bone Miner Res. 1996. 11(5): 568-577.
Additional References
27. Kappis M. Weitere beitrage zur traumatisch-mechanischen entstehung der “spontanen” knorpela biosungen. Dtsch Z Chir. 1922;171:13-29.
28. Konig F. Uber freie Korper in den gelenken. Dtsch Z Chir. 1888;27:90-109.
29. Brage ME, Bugbee W, Tontz W. Intraoperative and postoperative complications of fresh tibiotalar allografting. Presented at the annual Winter Meeting of the American Orthopaedic Foot and Ankle Society, Dallas, February 16, 2002.
30. Whittaker JP, Smith G, Makwana N, Roberts S, Harrison PE, Laing P, Richardson JB. Early results of autologous chondrocyte implantation in the talus. J Bone Joint Surg Br. 2005;87(2):179-183.
Editor’s note: For related articles, see “A Stepwise Approach For Osteochondral Lesions Of The Talus” in the May 2013 issue of Podiatry Today or the June 2011 DPM Blog, “Key Insights On Osteochondral Lesions Of The Talus,” by William Fishco, DPM, FACFAS.











